
NITROGEN - PHOSPHOROUS - POTASSIUM
In the last issue I discussed the factors affecting nutrient uptake, the effects of pH and other Issues. This time I would like to look at the 'big three'. That is, Nitrogen (N), Phosphorus (P) and Potassium (K). These are the most quoted elements of fertilisers and we will look at their functions and availability in common fertilisers. We will also compare the availability of these nutrients in relation to organic and inorganic fertilisers.
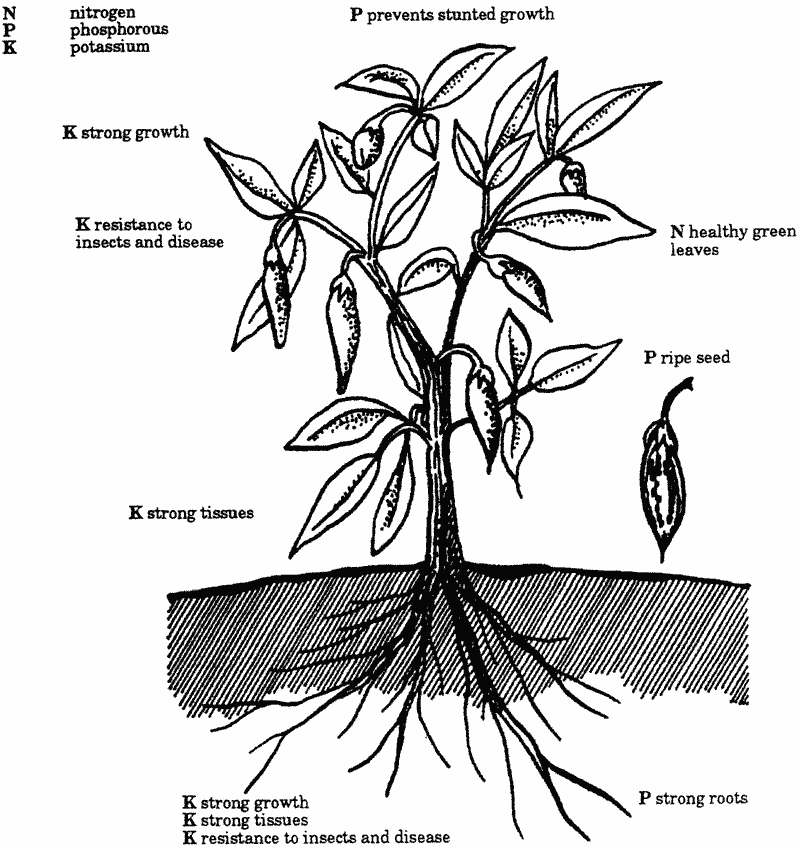
Let's look at the primary function of these elements within the plant structure.
Nitrogen is probably the most understood of the major elements in terms of needs and application.
Nitrogen is a major component of the plant's chlorophyll. Chlorophyll is a compound used by the plant as part of photosynthesis.
In this process, the sun provides energy to the chlorophyll to split water molecules into hydrogen and oxygen molecules. The hydrogen is then combined with carbon dioxide to form sugars that are stored as starch or used in other parts of the plant. Oxygen is then a by-product of this process and it is expelled back into the atmosphere.
This is where the plants gain their reputation as the 'lungs of the planet'. The greater the leaf/stem area, the greater need the plant has for nitrogen. The same applies to the plant's growth rate. More vigorous plants require more energy and therefore larger amounts of nitrogen.
Phosphorus is another vital building block in the plant's structure. Phosphorus is used in the nucleus of the plant cell.
It is also essential for strong root growth and the setting and ripening of seed. Sometimes this seed comes in a survival capsule, usually called 'fruit'.
Because of its role in plant cell nuclei, phosphorus must be available to the plant as it germinates and begins the rapid growth phase of its life. As the plant grows, it continues to require phosphorus in adequate amounts to reach maturity and seed set.
A lack of phosphorus may, in fact, lead to a plant never achieving the maturity to set and ripen fruit/seeds.
Potassium is the last of the major elements required. Potassium plays no part in the physical structure of a plant.
It has a vital role in the production of the sugars and starches as part of the photosynthetic process as well as proteins manufactured by other processes within the plant.
The presence of potassium in plant cells also strengthens the plants' defences against pest attack.
The amount of potassium available will also affect the flavour and colour of fruit. Potassium is less available as pH levels fall (i.e. the soil becomes more acidic).
Diagram 1 highlights the functions of the major elements.
Fertilisers may also contain a number of secondary and minor trace elements. These will be discussed in a later article.
The N, P and K content of inorganic fertilisers (that is, fertilisers manufactured by the chemical industry) is usually expressed as a ratio on the packaging.
The table will look something like this:
| N- | P- | K |
| 15.3- | 4.0- | 11.7 |
Ratios refer to the amount of that element available as a percentage by weight. Where a fertiliser is 'incomplete', the missing element will be denoted by a 0 in the ratio. For example:
| N- | P- | K |
| 16- | 0- | 10 |
Such precise ratios of these elements can only be achieved in a controlled chemical process.
Organic fertilisers can be analysed, but the ratios can vary widely depending on the age of the material, animal diets if it is a manure, and many other factors.
Table 1 illustrates the NPK of common brand fertilisers as well as animal manures.
We all should be aware of the potential hazards of handling fertilisers. Inorganic fertilisers should be handled wearing gloves. Some compounds can cause skin irritation and burning. Organic fertilisers are a often a manure product and may carry harmful bacteria. Gloves and a simple dust mask are recommended. It is also recommended that your Tetanus vaccination is up to date.
Table 1 Average composition of stored animal manures at 40 to 60% moisture.
| One tonne (1000 kg) of manure contains | ||||||
|---|---|---|---|---|---|---|
| Nitrogen (kg) | Phosphorus (kg) | Potassium (kg) | ||||
| range | average | range | average | range | average | |
| Horse | 7 to 12 | 9 | 5 to 9 | 7 | 4 to 13 | 5 |
| Cow | 8 to 11 | 9 | 5 to 8 | 6 | 4 to 13 | 7 |
| Sheep | 5 to 14 | 9 | 4 to 10 | 8 | 5 to 7 | 6 |
| Pig | 6 to 12 | 9 | 5 to 8 | 7 | 4 to 10 | 7 |
| Fowl | 8 to 26 | 18 | 6 to 20 | 13 | 4 to 12 | 7 |
| Note: To convert these figures to percentages, divide by 10. | ||||||
While products with all three major elements are common it is also possible to purchase fertilisers that are one element specific. Table 2 provides a handy reference for these element specific types.
In the next article I will look at the secondary elements as well as trace elements. I will also discuss application techniques and timing.
Types of fertilisers
| TYPES OF FERTILISERS | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type Single Fertilisers | Elements High N Moderate N Low N High P Low P High K | Fertiliser Urea Nitram Ammonium sulphate Super King Superphosphate Muriate of Potash Sulphate of Potash | % by Weight 46 N 34 N 20.5 N 19.2 P 9.2 P 50K 42 K | |||||||||||||||
| Compound Fertilisers | V. High N, High P High N, High P High N, V. High K | DAP MAP Nitrate of Potash | 19 N - 20 P 12 N - 22 P 13 N - 0 P - 38 K | |||||||||||||||
| Mixtures | High N, High P, High K V.High N,Low P, High K High N, Low P, Low K Low N, Low P, Low K | Crop King 55 Crop King 88 Tropic Q5 |
| |||||||||||||||
| Organic | Low N; very low P | Bio crop Dynamic Lifter | 4.4 N - 1.1 P - 0.2 K | |||||||||||||||
DATE: November 1998
* * * * * * * * * * * * * * *
